tin electron configuration|Tin : Tagatay The electron configuration shows that the last shell of tin has four electrons. Therefore, the valence electrons of tinare four. . Tingnan ang higit pa No other sex tube is more popular and features more Pinay Scandal scenes than Pornhub! Browse through our impressive selection of porn videos in HD quality on any device you own. . (Pinay Trending 2024) Missladygirl00. 34.3K views. 84%. 54 years ago. 7:11. Part - 2 Kantutan sa Phone - SARAP TALAGA😋😋😋 . MsPinaySarap. 6 .
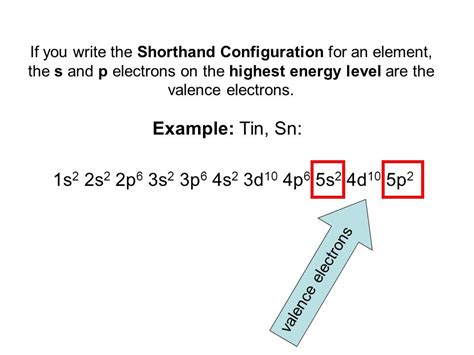
tin electron configuration,Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground-state electron configuration of tin is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. In the tin ground-state electron configuration, the last electrons of the 5p orbital are located in the . Tingnan ang higit patin electron configurationThe total number of electrons in tin is fifty. These electrons are arranged according to specific rules in different orbitals. The arrangement . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paThe electron configuration shows that the last shell of tin has four electrons. Therefore, the valence electrons of tinare four. . Tingnan ang higit pa
Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit paTin Learn about the properties, reactions, isotopes, allotropes, and oxidation states of tin, a Group 14 element. Find out its electron configuration and how it forms . To write the configuration for the Tin (Sn) and the Tin ions, first we need to write the electron configuration for just Tin (Sn). We first need to find the number of .Learn about tin, a soft, pliable metal with the electron configuration [Kr] 4d 10 5s 2 5p 2. Find out its history, uses, biological role, natural abundance and more. Tin Electron Configuration. It shows a chemical similarity to both of its neighbors that are germanium and lead in group 14 and has two main oxidation states, that is +2 and the little more stable +4. Aufbau principle. First, find electrons of tin atom. Periodic table | Image: Learnool. The atomic number of tin represents the total number of electrons of tin. Since the atomic number of tin is 50, the .
Tin is a post-transition metal with atomic number 50 and chemical symbol Sn. Its electron configuration is [Kr] 4d 10 5s 2 5p 2, with valence electrons 4 and valency electrons 2,4. Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic structure. The chemical symbol for Tin is Sn. .

Tin. Full electron configuration of tin: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. indium ← tin → antimony. Tin, complete electron configuration.
Crystal Structure. Centered Tetragonal. History. Tin was first smelted in combination with copper around 3500 BC to produce bronze. The oldest artifacts date from around 2000 BC. Cassiterite, the tin oxide form of tin, .

Tin is a chemical element of the periodic table with chemical symbol Sn and atomic number 50 with an atomic weight of 118.711 u and is classed as a post-transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: 5s 2: 5p 2: Electrons per shell: 2, 8, 18, 18, 4: Valence electrons : 4: Valency .Tin. Full electron configuration of tin: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 2 indium ← tin → antimony. © 2009-2016 | www.prvky.com . Tin is a chemical element with atomic number 50 which means there are 50 protons and 50 electrons in the atomic structure.The chemical symbol for Tin is Sn. Electron Configuration and Oxidation States of Tin. Electron configuration of Tin is [Kr] 4d10 5s2 5p2. Possible oxidation states are +2,4. Electron Configuration. The .For example, the atomic number of Sodium is 11 and its electron configuration is 1 s 2 2 s 2 2 p 6 3 s 1; Electronic configuration of Tin:-Atomic number of Tin is 50, that is Tin has a total of 50 electrons. Since 1s orbital can hold a maximum of 2 electrons, so first 2 electrons will fill the 1s orbital, and the next 2 electrons will fill the . To find the number of valence electrons for Tin (Sn) we need to look at its electron configuration. This is necessary because Sn is a transition metal (d bl.
Electronic configuration of the Tin atom. Valence electrons. Orbital diagram. Tin electron configuration. ← Electronic configurations of elements . Sn (Tin) is an element with position number 50 in the periodic table. Located in the V period. Melting point: 232 ℃.Element Tin (Sn), Group 14, Atomic Number 50, p-block, Mass 118.710. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.Electron Configuration of Tin. Tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form covalent tin (II) compounds with its two unpaired p-electrons. In the three dimensional figure below, the first and most inner electron shell is represented by blue electrons, the second electron shell made .
Tin atoms have 50 electrons and the shell structure is 2.8.18.18.4. The ground state electron configuration of ground state gaseous neutral tin is [ Kr ]. 4d 10 . 5s 2 . 5p 2 and the term symbol is 3 P 0 .tin electron configuration Tin This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at least 8 steps to determine the electron configuration, starting with finding the atomic number by looking at the list of orbitals and understanding the notation.. But wait .For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration . tin: [Kr] 5s 2 4d 10 5p 2. c) lead: [Xe] 6s 2 4f 14 5d 10 6p 2. 2. Scenario: You are currently studying the element .
Electron atomic and molecular orbitals A Bohr diagram of lithium. In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. [1] For example, the electron configuration of the neon atom is 1s 2 2s 2 2p 6, meaning that the 1s, 2s, and .Protons and Neutrons in Tin. Tin is a chemical element with atomic number 50 which means there are 50 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is .The valence electrons (here 3s 2 3p 3) are written explicitly for all atoms. Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule. Tin - Sn, on the periodic table is found in the fourteenth column of the periodic table Group IVB this is the second column of the p block. Tin is in the fifth energy level (row). This means that Tin must end with an electron configuration of #5p^2# The total electron configuration would be #1s^2 2s^2 2P^6 3s^2 3p^6 4s^2 3d^10 4p^6 . The electron configuration of tin is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2. Learn how to find: Tin electron configuration. Now in the next step, start drawing the orbital diagram for tin. Draw orbital diagram. Before drawing the orbital diagram, you should know the three general rules.Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 .La configuration électronique de Tin est 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. L'étain est l'élément chimique du tableau périodique qui appartient au groupe 14, son numéro atomique est 50 et son symbole est Sn.
tin electron configuration|Tin
PH0 · Tin, electron configuration
PH1 · Tin – Electron Configuration and Oxidation States – Sn
PH2 · Tin electron configuration
PH3 · Tin Electron Configuration (Sn) with Orbital Diagram
PH4 · Tin (Sn)
PH5 · Tin
PH6 · Electron Configuration for Sn, Sn 2+, and Sn 4+
PH7 · Complete Electron Configuration for Tin (Sn, Sn2+, Sn4+)
PH8 · Chemistry of Tin (Z=50)